Understanding their safety characteristics is paramount when it comes to handling, storing, and utilizing flammable liquids. Among the essential parameters to consider are a substance’s Flash Point and Fire Point. Though these terms often appear interchangeably, they represent distinct critical concepts in various industries, including petrochemical, transportation, and safety regulation. The Flash Point and Fire Point define different stages of a substance’s ability to ignite and sustain combustion, and each has unique implications for safety, handling, and regulatory compliance.
This blog aims to demystify these vital terms, exploring the differences between Flash Point and Fire Point, their significance, and the methods used to determine them. By comparing them, we’ll provide a comprehensive understanding that can inform safer practices and more robust safety protocols. Whether you’re a professional in the field, a student, or simply interested in the science behind flammable substances, this exploration into the Key Differences Between Flash Point and Fire Point will equip you with valuable insights and knowledge.

What is Flash Point?
The flash point of a volatile material is the lowest temperature at which it can vaporize to form an ignitable mixture in the air. In other words, it is the temperature at which the vapors of a liquid can “flash” or briefly ignite when an ignition source like a flame or spark is introduced.
It’s crucial to note that the flash point is not the temperature at which the liquid itself ignites, only the temperature at which the vapors it produces can ignite. The vapors cease to burn as soon as the ignition source is removed unless the temperature has reached the fire point; at this point, the vapor can continue to burn even after removing the ignition source.
The flash point is a key property for safety considerations, as it indicates the risk of a liquid causing fire or explosion. Materials with lower flash points pose a higher fire risk and require more care in handling and storage.
There are two primary methods for testing the flash point of a substance:
- Open Cup Method: In this method, the sample is heated in an open cup, and an ignition source is passed over the top at regular intervals until the vapor ignites.
- Closed Cup Method: The sample is heated in a closed cup, and the ignition source is introduced into the vessel. The closed cup method generally gives a lower flash point and is more widely accepted as it better mimics real-world conditions.

What is Fire Point?
The fire point is the temperature at which a liquid’s vapors will continue to burn for at least five seconds after ignition by an open flame or other ignition source. It is closely related to the flash point but is a higher temperature.
While the flash point is the lowest temperature at which vapors of a liquid can ignite, the fire point is concerned with the ability of those vapors to sustain continuous burning. At the fire point, the vapor/air mixture is rich enough, and conditions are such that the combustion will continue even after the ignition source is removed.
The fire point is particularly relevant when assessing the fire risk of oils and other combustible liquids. It provides information about how a fire might behave once ignited and helps determine safety measures for handling and storing the material.
Typically, the fire point is measured using an open cup apparatus, which is also used to determine the flash point. The liquid to be tested is heated, and a small flame is passed over the surface. The temperature at which the burning becomes continuous, rather than just flashing and going out, is recorded as the fire point. In general, the fire point of a substance is about 10°C higher than its flash point, although this can vary depending on the specific material and conditions.
Understanding the fire point of a substance is important in various industrial applications, including the selection and design of equipment, as well as the implementation of safety protocols for handling, transporting, and storing flammable or combustible liquids.
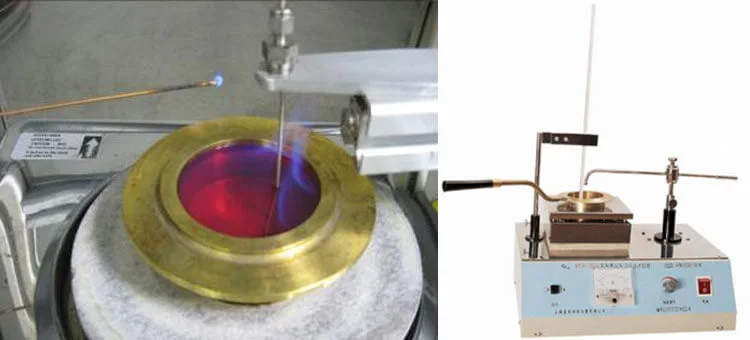
Key Differences Between Flash Point And Fire Point
The flash point and fire point are two important characteristics related to flammable liquids, and they are used to determine the fire risk associated with a particular material. Here are the key differences between flash point and fire point:
| Aspect | Flash Point | Fire Point |
|---|---|---|
| Definition | Lowest temperature where vapors can ignite but not sustain burning. | Lowest temperature where vapors ignite and continue to burn for at least five seconds. |
| Temperature | Generally lower | Usually higher, often about 10°C above the flash point |
| Burning Continuity | Vapors ignite but do not continue to burn | Vapors continue to burn even after ignition source is removed |
| Testing Method | Can be determined using open or closed cup methods; observes momentary ignition | Can be determined using open or closed cup methods; observes continuous burning |
| Significance | Used for classifying materials, handling, storage, transportation | Relevant for understanding fire behavior, fire-fighting strategies |
| Regulatory and Safety Considerations | Commonly used in regulations for safety protocols | Less commonly used in regulations but informs safety measures in specific settings |
Definition:
Flash Point: The lowest temperature at which the vapors of a liquid can form an ignitable mixture in the air and can be ignited by an external source (such as a flame or spark), but cannot sustain the burning.
Fire Point: The lowest temperature at which the vapors of a liquid not only ignite but continue to burn for at least five seconds after the ignition source is removed.
Temperature:
The temperature at which the fire point occurs is generally higher than the flash point. This is because, at the fire point, enough vapors are produced to sustain the combustion, whereas, at the flash point, the combustion will not. The temperature difference can provide insights into how quickly a material might transition from ignition to sustained burning.
Burning Continuity:
- Flash Point: At the flash point, there’s a brief moment of ignition, but the vapors do not continue to burn. This illustrates that the concentration of vapors is at the minimum threshold for ignition but not sufficient to sustain combustion.
- Fire Point: The vapors continue to burn at the fire point, indicating a higher concentration of combustible vapors in the air. This sustained burning helps to differentiate between materials that may ignite briefly and those that can sustain a fire.
Testing Method:
Flash and fire points can be measured using open or closed-cup methods.
- Open Cup Method: Here, the liquid is exposed to atmospheric conditions. This method often results in higher readings, as the vapors are not contained.
- Closed Cup Method: The liquid is tested in a sealed container, and the ignition source is introduced into the vessel. This typically results in lower readings due to the containment of the vapors.

These methods emphasize the importance of standardization in testing, as different conditions can yield varying results.
Significance:
- Flash Point: It is used to categorize materials, helping to determine how they should be handled, stored, and transported. Knowing the flash point assists in risk assessment and informs safety procedures to prevent accidental ignition.
- Fire Point: While less commonly used for regulatory purposes, the fire point provides valuable insights into how a fire might behave once started. This information is valuable for firefighting strategies and can also guide design considerations in industrial settings.
Regulatory and Safety Considerations:
Understanding the flash and fire points is integral to maintaining safety standards. Regulations often reference the flash point to classify hazardous materials, whereas the fire point might be used to create tailored safety procedures in specific industries or applications.
In summary, flash points and fire points play vital roles in understanding the flammability characteristics of materials, but they describe different phases of the combustion process. Knowing these parameters is essential for ensuring safe handling and use of flammable substances.
Conclusion
Understanding the key differences between Flash Point and Fire Point is vital for anyone dealing with flammable materials, as it informs safe handling, storage, and utilization practices. While Flash Point represents the lowest temperature at which a liquid’s vapors can ignite but not sustain combustion, the Fire Point is when the vapors continue to burn even after the ignition source is removed. These distinctions have profound implications in various industries and safety regulations, guiding the classification of materials and the creation of safety protocols.
By appreciating the unique characteristics of Flash Point and Fire Point, professionals and enthusiasts alike can foster a safer environment, optimize performance in industrial applications, and contribute to the responsible management of potentially hazardous substances.

